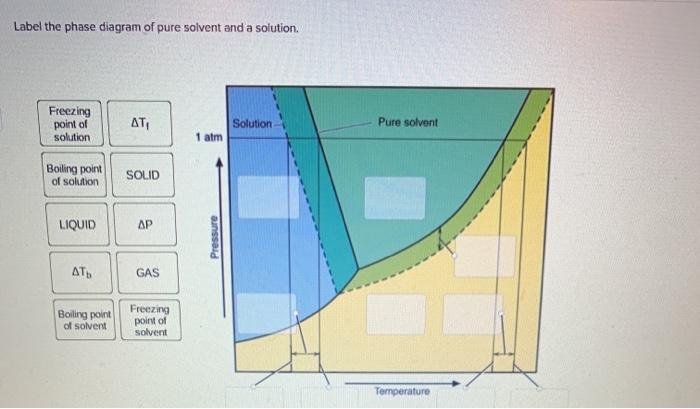
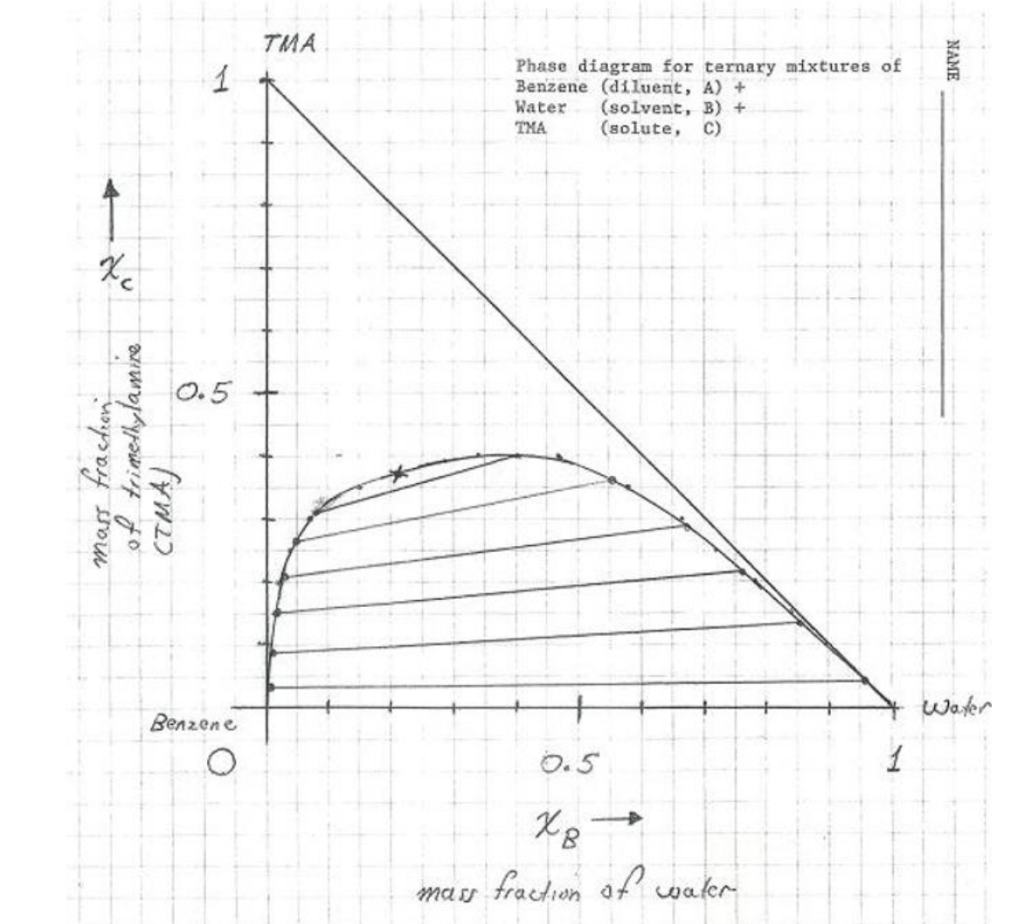
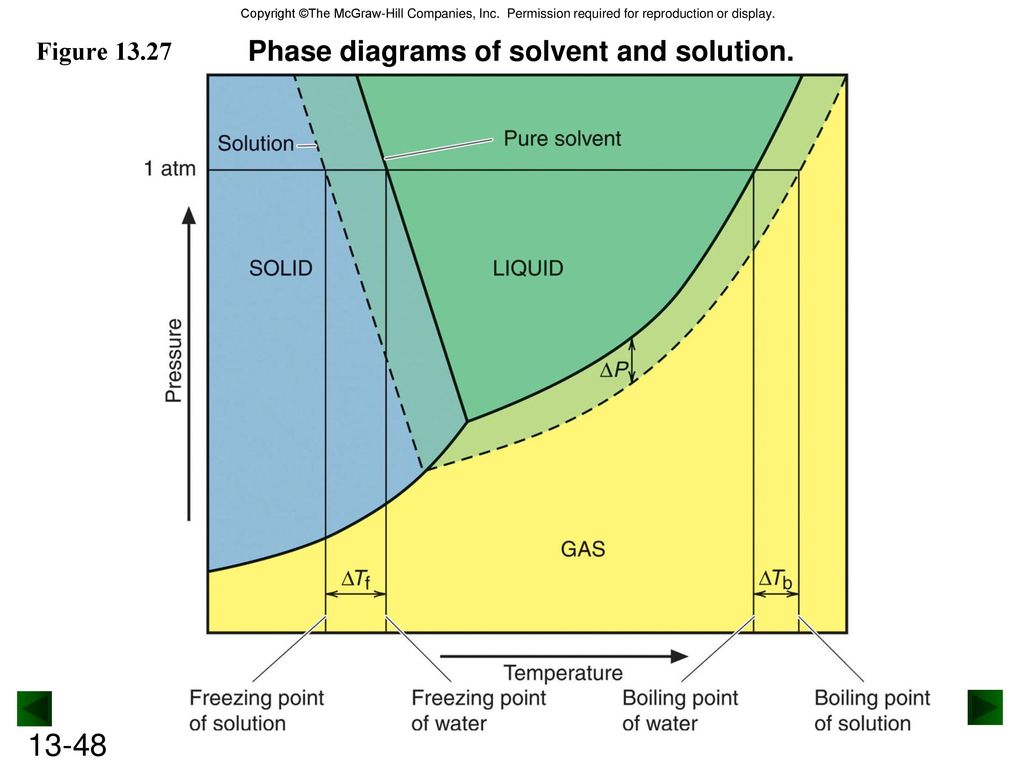
41 label the phase diagram of pure solvent and a solution.
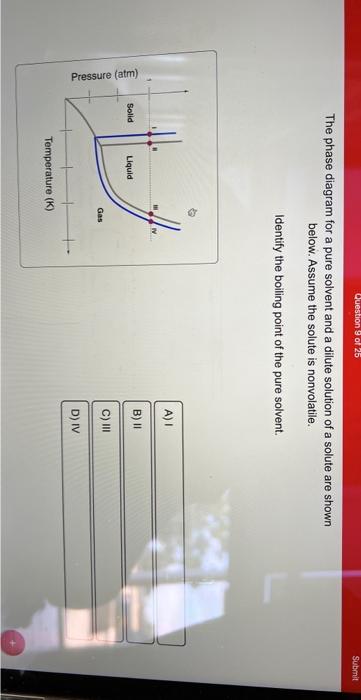
Phase and Phase System (Solution Solvent Solute) If a small amount of sugar is added to this sample of water, the sugar dissolves but a sample remains as a single liquid phase. However, the properties and composition of this new liquid phase, now the sugar solution, are different from those of pure water. As this solution of sugar in water is containing two substances (binary solution), so it ... The figure shows two phase diagrams, one for a pure liquid (black line ... The figure shows two phase diagrams, one for a pure liquid (black line) and the other for a solution made using the liquid as the solvent (red line). What does point B represent? Chemistry Phases of Matter Phase Diagrams 1 Answer Truong-Son N. Jun 10, 2017 Consider the following general phase diagram:
The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute Doubtnut 2.5M subscribers 522 views 2 years ago The phase diagrams for the pure solvent (solid...

Label the phase diagram of pure solvent and a solution.
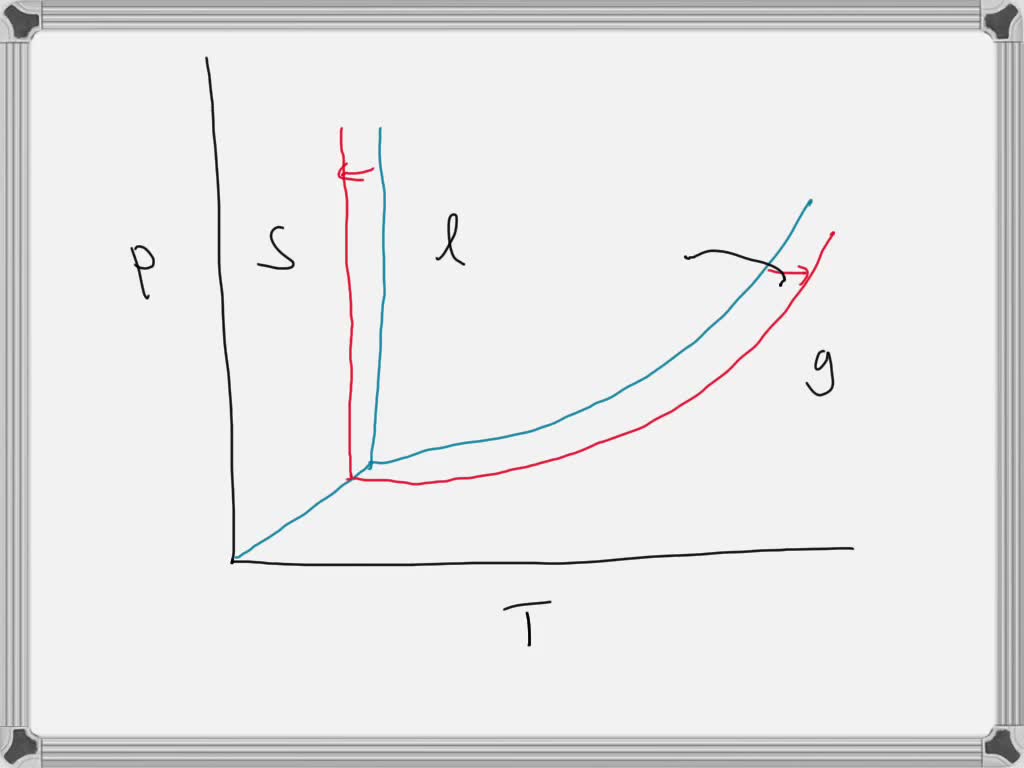
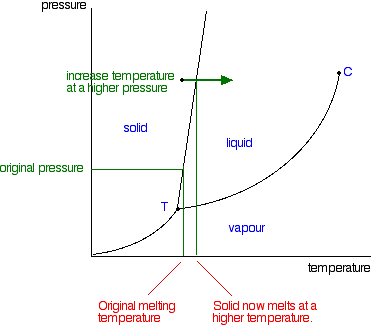
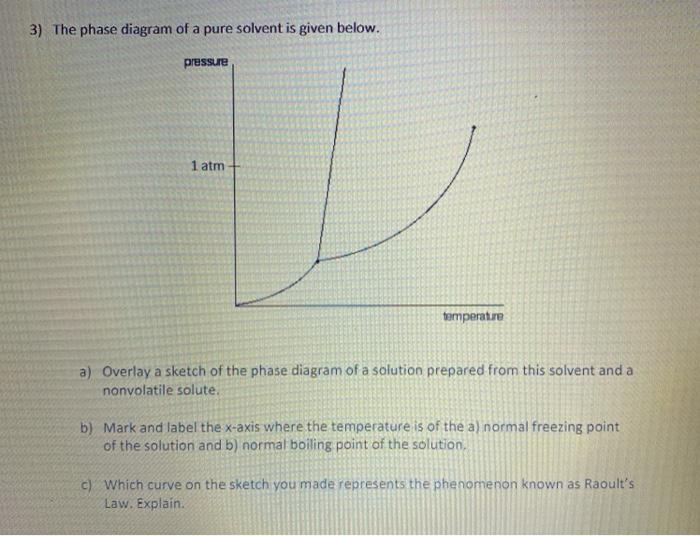
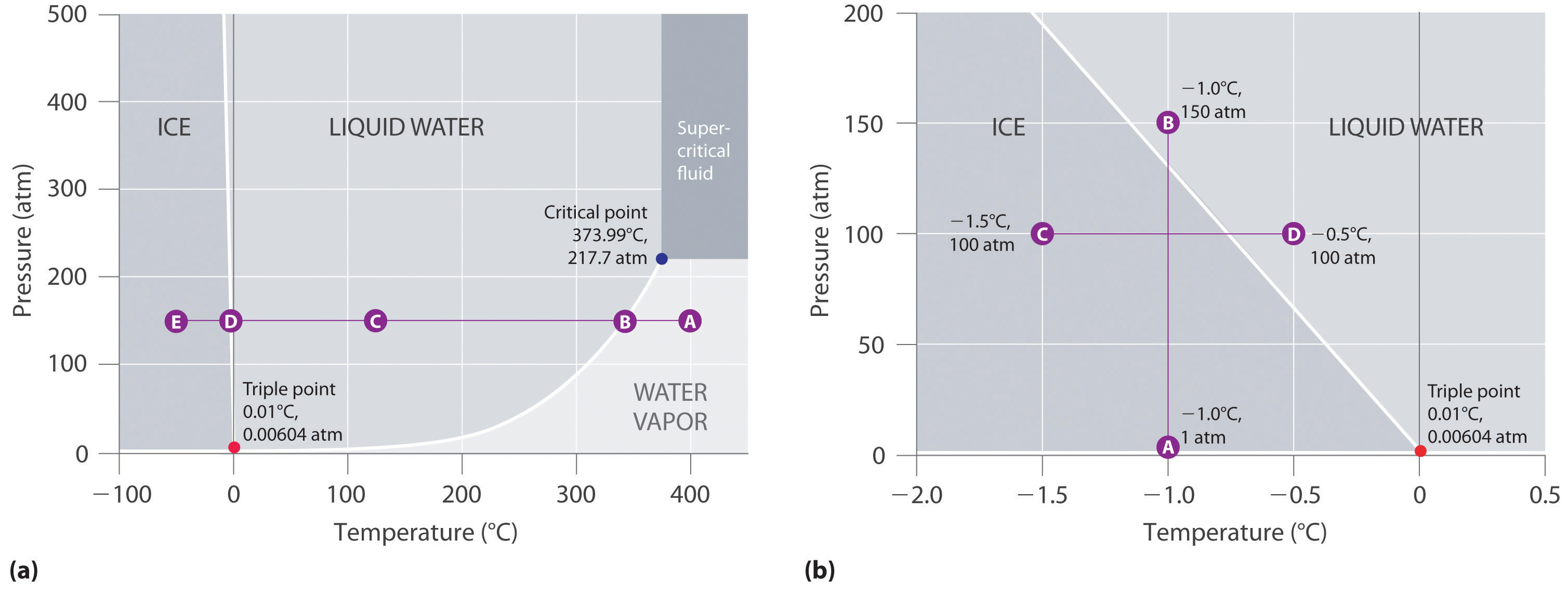
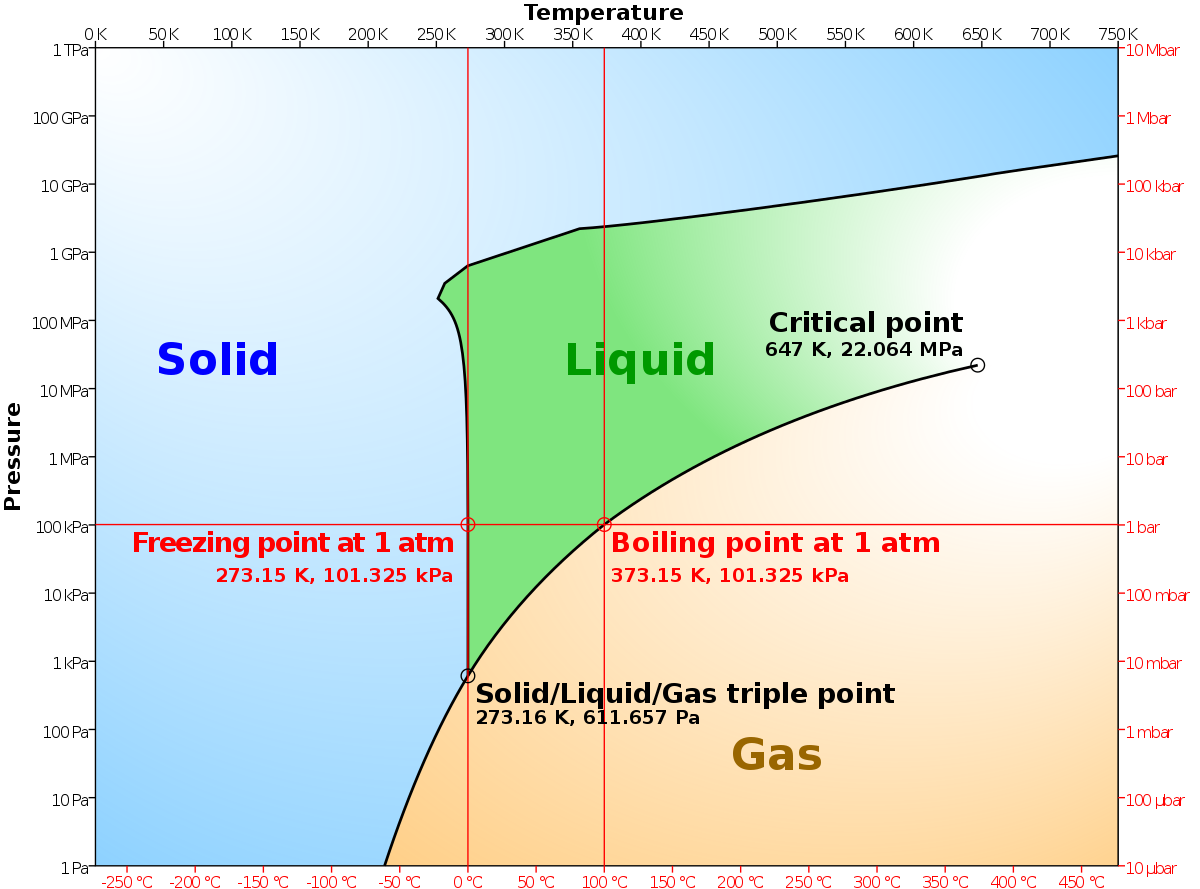
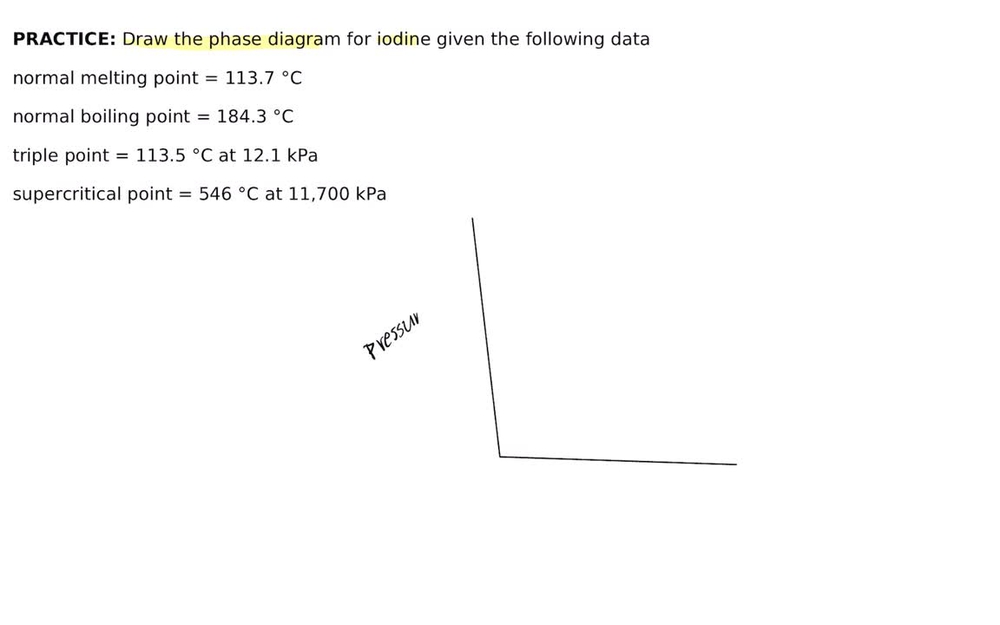
phase diagrams of pure substances - chemguide A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. (Get Answer) - Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature Posted 10 months ago Recent Questions in Chemistry Q: (Get Answer) - Label the phase diagram of pure solvent and a solution ... Expert's Answer. Phase Behaviour of t-Butane. Summarize the pressure/temperature combinations for the triple,... 7) Phase Diagram Label the following Solid. Liquid, Gas, Triple Point, Critical Point, Sublimation Curve, Vapor Pressure Curve, Fusion Curve 150 T.°c The approximate normal boiling point of this substance is The approximate normal ...
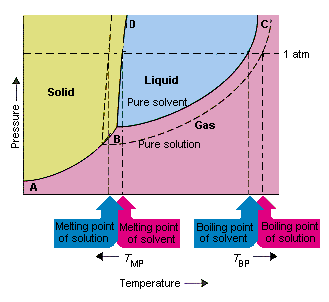
Label the phase diagram of pure solvent and a solution.. Answered: The phase diagrams for a pure solvent… | bartleby Transcribed Image Text:The phase diagrams for a pure solvent and the solvent in a solution are shown. (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm Liquid Solid Label The Phase Diagram Of Pure Solvent And A Solution. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal. Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, Boiling & Critical Point A niko commission by vallsmaxime. 7+ label the phase diagram of pure solvent and a solution Label the phase diagram of a pure solvent and a solution. In the cases well be looking at on this page the phases will simply be the solid liquid. Welcome back or working on Chapter thirteen Problem ton and were looking at the following diagram that shows a vapor pressure curves of both a volatile solvent and a solution of a. What are the most important differences between the phase di | Quizlet What are the most important differences between the phase diagram of a pure solvent and the phase diagram of a solution containing that solvent? Solution Answered 1 year ago Create an account to view solutions chemistry Express Raoult's law in words. Is Raoult's law valid for a solution of a volatile solute? Explain. chemistry
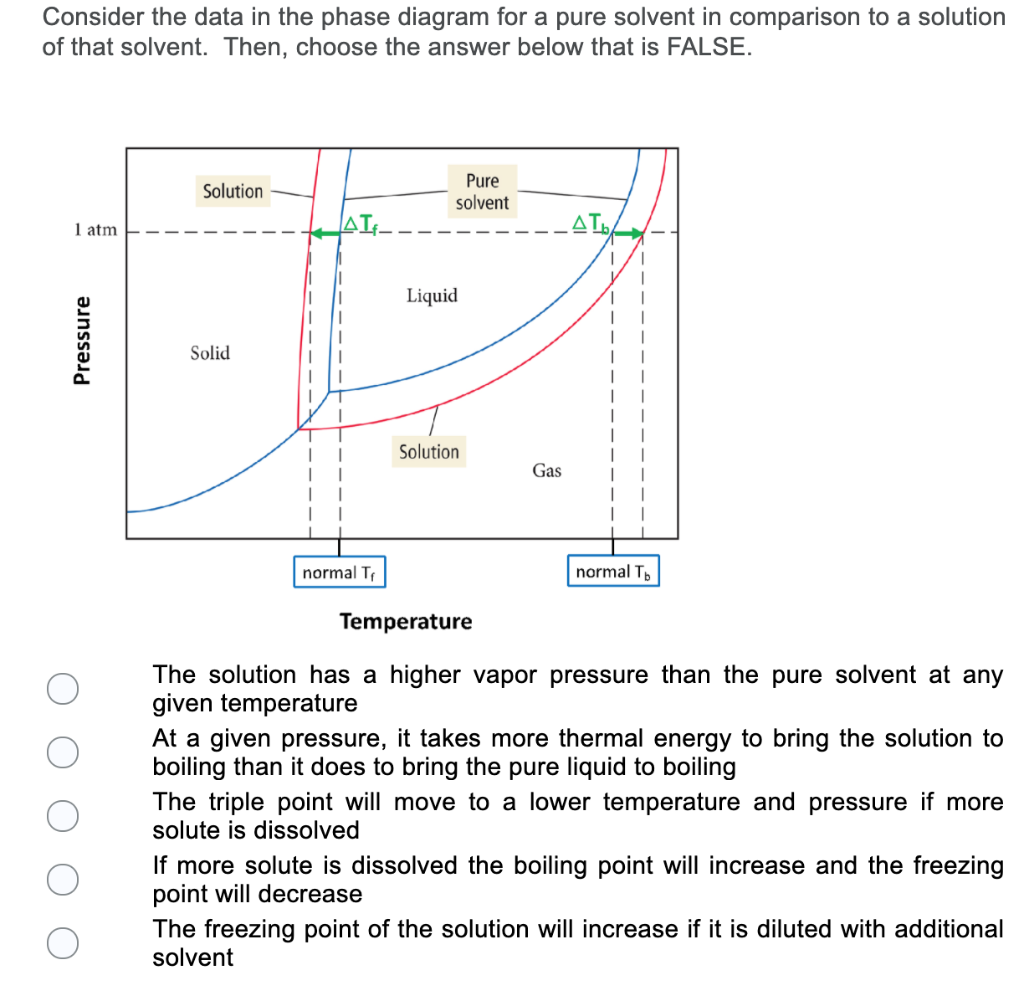
The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm. Phase Diagrams - Chemistry LibreTexts Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ... The following phase diagram shows part of the liquid-vapor phase ... The following phase diagram shows part of the liquid-vapor phase-transition boundaries for two solutions of equal con- centration, one containing a nonvolatile solute and the other containing a volatile solute whose vapor pressure at a given temperature is approximately half that of the pure solvent. 13.6: Vapor Pressures of Solutions - Chemistry LibreTexts We can understand this phenomenon qualitatively by examining Figure 13.6.1, which is a schematic diagram of the surface of a solution of glucose in water. In an aqueous solution of glucose, a portion of the surface area is occupied by nonvolatile glucose molecules rather than by volatile water molecules.
A phase diagram for solvents and solutions is shown in the figure. What ... Hint: We are discussing the normal boiling point of the solution. We should know first what the normal boiling point is. Normal boiling point of a solution is the temperature at which the vapour pressure of the solution becomes equal to the atmospheric pressure. We are given the phase diagram for solvents and solutions. 13.8: Freezing-Point Depression and Boiling-Point Elevation of ... Figure 13.8.1: Phase Diagrams of Pure Water and an Aqueous Solution of a Nonvolatile Solute. The vaporization curve for the solution lies below the curve for pure water at all temperatures, which results in an increase in the boiling point and a decrease in the freezing point of the solution. Phase Diagrams for Pure Substances - Chemistry LibreTexts A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance. This is the phase diagram for a typical pure substance. Draw a phase diagram showing how the phase boundaries differ for a pure ... Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution. Channels. Recent Channels. General Chemistry; Chemistry. ... This video solution was recommended by our tutors as helpful for the problem above. 94 views. Was this helpful? 0.
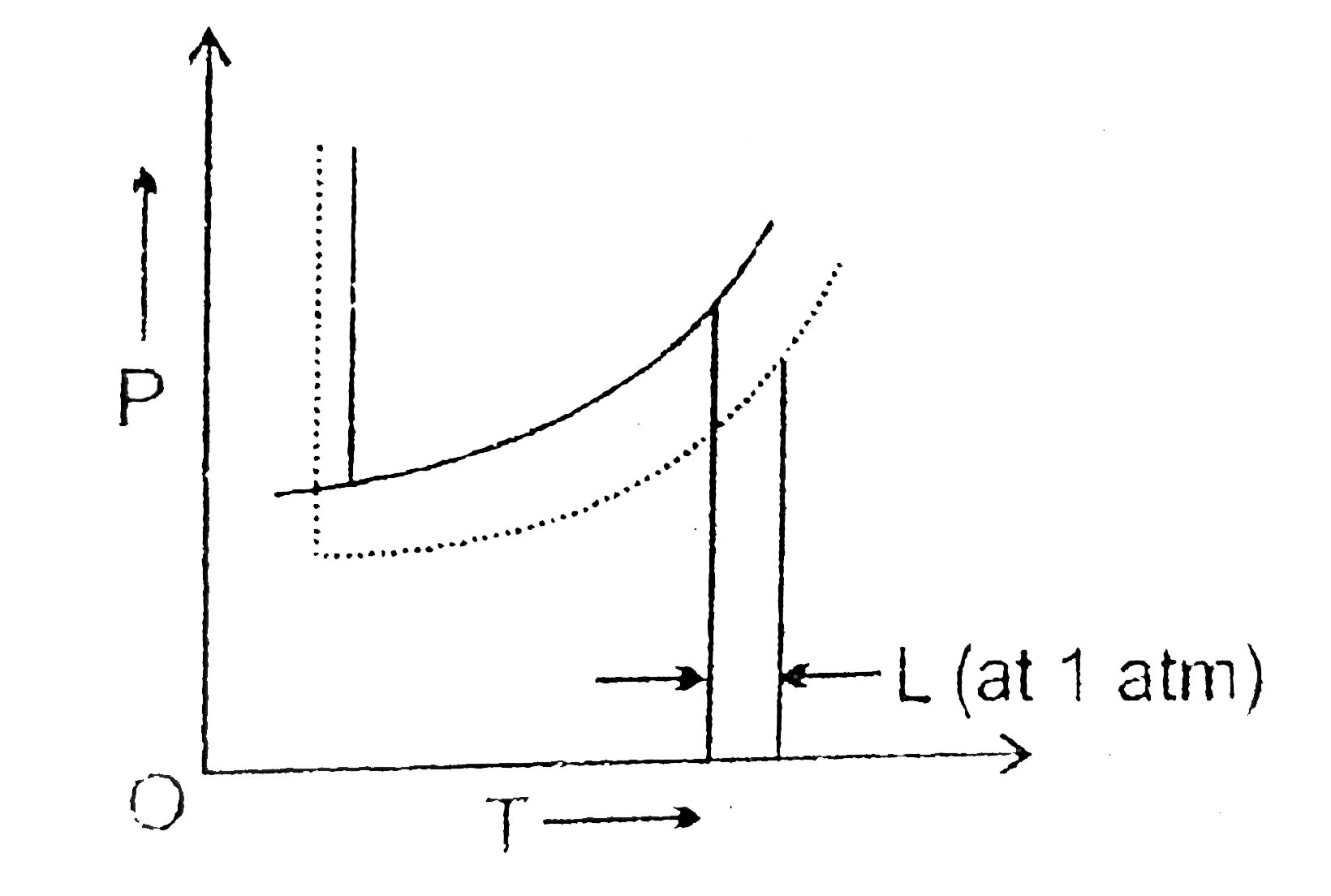
label the phase diagram of pure solvent and a solution The phase diagrams for the pure solvent solid lines and the solution non-volatile solute dashed line are recorded below. Label the phase diagram of pure solvent and a solution. The quantity indicated by L in the figure is. Summarize the pressuretemperature combinations for the triple freezing and boiling points for t-butane and use these values ...
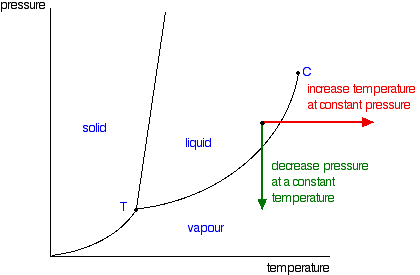
Draw a phase diagram showing how the phase boundaries differ for a pure ... As we can observe from the diagram, the liquid/vapor phase transition line, is at a lower temperature and as a result the triple point is also lower, thus: the solution \blue{\text{solution}} solution will have a lower freezing point compared to the pure solvent \orange{\text{pure solvent}} pure solvent.
Label the phase diagram of a pure solvent and a solution. Label the phase diagram of a pure solvent and a solution. + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. John Edward Cayas Lv10 16 Oct 2020 Unlock all answers Get 1 free homework help answer. Already have an account? Log in Like turquoiseelk355 is waiting for your answer Log in to answer + 20
[Solved] Label the phase diagram of pure solvent a | SolutionInn Label the phase diagram of pure solvent and a solution. Freezing point of solution Boiling point of solvent ATb AT₁ Boiling point of solution GAS Freezing point of solvent SOLID ΔΡ LIQUID 1 atm Pressure Solution Pure solvent Temperature Expert Answer The dotted line indicates solution The linear line indicates pur View the full answer
Answered: What are the most important differences… | bartleby Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution. Give an explanation for the phase diagram. arrow_forward
Question: Label the phase diagram of pure solvent and a solution. Label the phase diagram of pure solvent and a solution. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Label the phase diagram of pure solvent and a solution. Show transcribed image text Expert Answer 100% (146 ratings) This is … View the full answer
Solved Label the phase diagram of pure solvent and a - Chegg Question: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature Show transcribed image text Expert Answer 100% (11 ratings) Transcribed image text:
The phase diagrams for = pure solvent and the solvent solution are shown. Identify the normal freezing ((pwolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and ...
Label The Phase Diagram Of Pure Solvent And A Solution A typical phase diagram has pressure on the y-axis and temperature on the x-axis. Figure 1: Example of a general phase diagram. The labels on the graph represent the physical state or phase of the substance at equilibrium. Feb 28, · Thus the boiling point of a solution is always greater than that of the pure solvent. We can see why this must ...
(Get Answer) - Label the phase diagram of pure solvent and a solution ... Expert's Answer. Phase Behaviour of t-Butane. Summarize the pressure/temperature combinations for the triple,... 7) Phase Diagram Label the following Solid. Liquid, Gas, Triple Point, Critical Point, Sublimation Curve, Vapor Pressure Curve, Fusion Curve 150 T.°c The approximate normal boiling point of this substance is The approximate normal ...
(Get Answer) - Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature Posted 10 months ago Recent Questions in Chemistry Q:
phase diagrams of pure substances - chemguide A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
















Post a Comment for "41 label the phase diagram of pure solvent and a solution."