43 open label study meaning
Open-label Definition & Meaning - Merriam-Webster open-label: [adjective] being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject — compare double-blind, single-blind. Open Label Extension Study Definition | Law Insider definition. Open Label Extension Study means the open label extension study being conducted by Rigel as of the Execution Date and as described in further detail in the Development Plan. Open Label Extension Study means, with respect to each Collaboration Program, the open label extension trial for such Collaboration Program described in the Pre ...
Open-label study - definition of Open-label study by The Free Dictionary Define Open-label study. Open-label study synonyms, Open-label study pronunciation, Open-label study translation, English dictionary definition of Open-label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
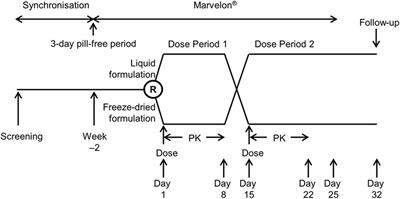
Open label study meaning
Open Label Study Definition | Law Insider Define Open Label Study. means the open label studies identified as *****. Meaning of "Open Label Pilot study"..?? - SAS Support Communities Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. Pilot study means a smaller version of a larger study that is conducted to prepare for that (larger) study. Pilot studies are used in clinical trials, in order to test different doses, routes of ... Open label study - definition of open label study by The Free Dictionary Define open label study. open label study synonyms, open label study pronunciation, open label study translation, English dictionary definition of open label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
Open label study meaning. NCI Dictionary of Cancer Terms NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Open label study definition and meaning - powerthesaurus.org Open label study definition based on common meanings and most popular ways to define words related to open label study. Open label study | definition of open label study by Medical dictionary open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Open-label study - definition of Open-label study by The Free Dictionary Open-label study synonyms, Open-label study pronunciation, Open-label study translation, English dictionary definition of Open-label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ... Understanding Clinical Trial Terminology: What is a Long-Term Extension ... Clinical trials are important research studies that are conducted to test potential new medicines in people after earlier stages of research have been successfully completed. Clinical trials typically move forward in a series from early-stage, small-scale Phase 1 studies to late-stage, large-scale, Phase 3 studies. These studies could be double-blind in which case the trial […] Open-label extension studies: do they provide meaningful information on ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ... Open-label study definition and meaning - powerthesaurus.org Open-label study definition based on common meanings and most popular ways to define words related to open-label study.
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation. Open label study - definition of open label study by The Free Dictionary Define open label study. open label study synonyms, open label study pronunciation, open label study translation, English dictionary definition of open label study. n. pl. stud·ies 1. Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. What is an open label extension study? - NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. This contrasts with single blind and double blind experimental designs, where participants are not aware of what treatment they are receiving (researchers are also unaware in a double blind ...
Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments.
PDF What Are Open-Label Extension Studies For? factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized
Open label study - definition of open label study by The Free Dictionary Define open label study. open label study synonyms, open label study pronunciation, open label study translation, English dictionary definition of open label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
Meaning of "Open Label Pilot study"..?? - SAS Support Communities Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. Pilot study means a smaller version of a larger study that is conducted to prepare for that (larger) study. Pilot studies are used in clinical trials, in order to test different doses, routes of ...
Open Label Study Definition | Law Insider Define Open Label Study. means the open label studies identified as *****.
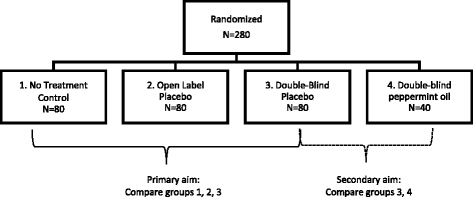


























Post a Comment for "43 open label study meaning"